Alright folks, let’s talk about something a bit more technical today. I stumbled upon this interesting process, something called Gas to Liquids (GTL). Now, I’m no chemist, but the potential applications seem pretty significant, so let’s dive in and see what we can learn.
The Gas to Liquids Process
Imagine turning natural gas – something we’ve got plenty of – into usable liquid fuels like gasoline or diesel. That’s the basic idea behind GTL. The diagram I saw breaks it down pretty clearly. It involves a series of steps, starting with the gas source and ultimately resulting in those sweet, sweet liquids we need to keep our cars running and our supply chains moving. Here’s a visualization:
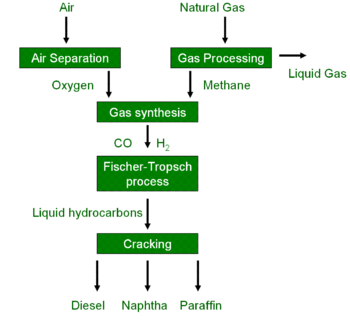
GTL Recipe Ingredients:
- Natural Gas (Methane - CH4): Our primary raw material. Think of this as the flour in our bread recipe. It’s the fundamental building block.
- Oxygen (O2): Essential for the initial oxidation or partial oxidation steps. Just like the yeast helps the bread rise, oxygen helps breakdown methane
- Catalyst (e.g., Cobalt or Iron-based): This is the secret ingredient! Different catalysts can be used for different stages, and they drastically affect the efficiency and type of liquid products produced. Think of it as your special spice blend that makes your chili better than everyone else’s.
- Steam (H2O): Often used in reforming processes to adjust the ratio of hydrogen to carbon monoxide.
GTL Recipe Instructions:
- Syngas Production: The first step involves converting the natural gas into syngas, a mixture of carbon monoxide (CO) and hydrogen (H2). There are a few methods for doing this, including steam reforming, partial oxidation, and autothermal reforming. Think of this as prepping your ingredients: turning a bunch of raw veggies into diced and ready-to-cook components.
- Fischer-Tropsch Synthesis: This is where the magic happens! The syngas is fed into a reactor containing the catalyst. Under specific temperature and pressure conditions, the carbon monoxide and hydrogen react to form long-chain hydrocarbons (paraffins and olefins). This is like the baking phase, where the ingredients interact to create the desired output.
- Upgrading: The hydrocarbons produced in the Fischer-Tropsch synthesis are typically not ready for direct use. They need to be “upgraded” through processes like hydrocracking and isomerization to improve their properties. This is like adding frosting and decorations to your cake to make it more palatable and visually appealing.
- Separation and Purification: Finally, the desired liquid fuels are separated from the mixture and purified. Unwanted byproducts are removed, and the final product is ready for distribution. This is the last step to ensure quality
Now, I understand this is a very simplified explanation. The actual process involves complex engineering and precise control of various parameters. But hopefully, this gives you a basic understanding of how GTL works. It’s an interesting alternative to traditional crude oil refining, and it could play a significant role in our energy future. Imagine if we could use stranded gas reserves, far from pipelines, to create useful fuels locally! It’s a fascinating area, and I’m curious to see how it develops further. What do you folks think? Have you heard much about GTL before?
If you are looking for Gas to liquids - Wikipedia you’ve visit to the right page. We have 1 Pics about Gas to liquids - Wikipedia like Gas to liquids - Wikipedia and also Gas to liquids - Wikipedia. Read more:
Gas To Liquids - Wikipedia

Gas to liquids. Gas to liquids